Use of computer navigation in total hip arthroplasty (literature review)
Use of computer navigation in total hip arthroplasty (literature review)
Published: 17.08.2022
Use of computer navigation in total hip arthroplasty (literature review)
Oleksandr A. Haluzynskyi1, Volodymyr S. Chornyi2, Svitlana V. Burburska1, Yevhenii V. Kozik1
1SI “INSTITUTE OF TRAUMATOLOGY AND ORTHOPEDICS OF NAMS OF UKRAINE”, KYIV, UKRAINE
2BOGOMOLETS NATIONAL MEDICAL UNIVERSITY, KYIV, UKRAINE
Wiad Lek. 2022;75(7):1765-1770
ABSTRACT
The aim: Analyze the accuracy and ease of use of various computer navigations in total hip arthroplasty.
Materials and methods: Data from about 50 literature sources for the last two decades have been analysed.
Conclusions: Analyzing the accuracy and ease of use of various computer navigations in total hip arthroplasty, we offer two the most promising for further study and improvement systems: a semi-active navigation system and augmented reality system in total hip arthroplasty.
KEY WORDS: hip arthroplasty, computer navigation, computer assisted orthopedic surgery
INTRODUCTION
Modern traumatology and orthopaedics are significantly concerned with developing and introducing effective surgery methods for the patients with diseases and traumas of the hip joint. According to the WHO, by 2050 twenty-five percent of population will be diagnosed with the bone and joint disorders [1]. Many authors suppose that the best, and sometimes the only treatment choice on late disorder stage is hip arthroplasty [2]. This surgical intervention restores the joint function, improves life quality and, regarding the working-age population, aids in their ability to work [3]. The operation is made for almost all age groups, from teenagers to the elderly and senile patients [4]. Nowadays about 1.5mln operations of hip arthroplasty are carried out annually [5]. The number of hip replacement interventions is still increasing, as about 572000 operations a year are predicted in the USA in the nearest time[6].
A successful total hip arthroplasty (THA) depends on such factors as the correct implant choice, optimum access, appropriate rehabilitation and postoperative management, with the endoprosthesis components correct position [7].
Throughout the endoprosthetics development surgeons and engineers have paid great considerable attention to the endoprosthesis design, ways of attachments and the materials as well as the surgical access. The late trend is represented with developing various methods of the correct and most “anatomical” implants alignment [8]. Berend K.R. et al. think that the correct alignment of the cup during hip arthroplasty is the most important factor for the desired operation success [9]. Correct implant alignment is a difficult task both with the primary endoprosthetics (in simple and complicated cases) and the revision interventions.
The hardest is location of endoprosthesis components in bone tumour operations or interventions related to previous surgical errors, with significant bone defects or absence of bone reference points [10]. Incorrect implant position may in short-term perspective result in the endoprosthesis head dislocation, and in the long-term perspective it affects wearing out of the inlay and implant functional capacity. Correct cup alignment helps to avoid early aseptic instability and dislocation of endoprosthesis.
In 1978 G.E. Lewinnek wrote about the so-called “safe zone”, aligning the acetabular component within the region, the dislocation risk is minimised: 40 ± 10 °, inclination, 15 ± 10 ° anteversion [11].
Various authors suggested different cup position with the THA, particularly: vertical inclination angle 35 °-45 °[12]; 45 ° ± 5 °; 20° -30° in males and 45° in females [13]; 25 °-45 °[14]; 25°-50° [15]. The anteversion angle was suggested equal to 0 -10 °[13]; 15 ° ± 5 ° [16]; 10 ° -20 ° [17]. Some authors state that the so-called combined anteversion is a more important parameter than only anteversion of the endoprosthesis acetabular component [18].
The acetabular component is affected by the pelvic tilt, i.e., an angle between the anterior pelvic plane and frontal of the patient [16,19], depending on the patient’s posture. The safe zone (Lewinnek) doesn’t consider sagittal pelvic mobility. So, the authors use a new term “functional acetabular component alignment” [20]. The functional alignment of the acetabular component is represented with its alignment regarding the sacral-pelvic balance. According to Lembeck and authors, pelvic tilt of 1 ° will lead to functional cup anteversion by 0.7° [21]. The studies of Dorr et al. have shown that each new increase of the anterior pelvic tilt by 1 ° will cause decrease in the acetabulum anteversion by 0.7 °– 0.8° [15]. The authors, having studied pelvic motion, concluded that the pelvic tilt should be regarded during the acetabular component implantation, with no permanency of anteversion angle. The anteversion and inclination angle, measured on the post-operative roentgenograms, depend on the position of the patient: recumbent and upright [22]. Here it becomes obvious that after the hip bone alignment, the pelvic tilt changes are comparable to its initial ones [23].
All this proves relevance of studying the computer navigation for alignment and precise implant alignment during the THA by confirming its position basing on intra-operational and quantitative assessment in real time. The computer-assisted orthopaedic surgery is a direction of orthopaedic surgery, used for correct positioning of surgical instruments during the operative intervention.
THE AIM
Analyze the accuracy and ease of use of various computer navigations in total hip arthroplasty.
MATERIALS AND METHODS
Analysis of 49 literature sources contains information about the main errors in the identification of components of the endoprosthesis, which may be related to the human factor, pelvic tilt and other reasons, and proves relevance of using computer navigation in different countries. The literature review contains the analysis of original articles and sources in four main scientometric databases: PubMed, Scopus, Web of Science.
REVIEW
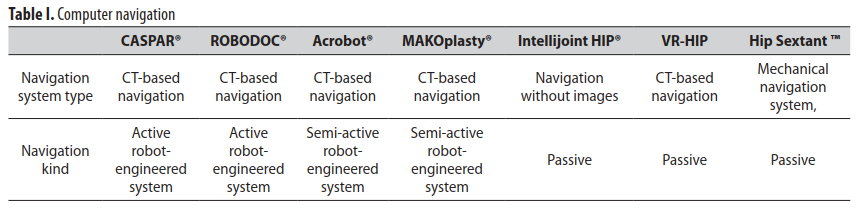
The CAOS(Computer Assisted Orthopedic Surgery) systems are divided into passive, active, and semi-active systems, according to the used surgical facilities and way of their functioning (Table I.). Passive systems, such as navigation, help operator in pre-operative planning as well as in informing about implant position during the operation, without his active participation in surgery. Active systems include surgical robots, which autonomously perform different surgery stages, scheduled by the operator. Semi-active systems use the implant computer alignment, which was planned before the operation. Navigation system, based on augmented reality, is also widely used.
The following navigation systems are used in orthopaedics: navigation without images, CT-based navigation, X-ray-based navigation. The image-based systems usually use pre-operation tomography in order to increase intra-operation registration. The other authors also describe methods of visualization, including fluoroscopy and ultrasonography [24].
PASSIVE CAOS SYSTEMS
With these systems used, pins are inserted into the pelvis and hip bone, where the marker spheres are attached. Information from the marker spheres on three-dimensional bone parameters is transmitted to the personal computer using infrared rays [39]. The screen shows a virtual model of the pelvic bones, hip bone, instruments and the information required for aligning the implants. To define position of the pelvic and hip bones one should attach a special indicator to appropriate protruding bone points.
In order to provide for the correct endoprosthesis alignment, the surgeon should use various anatomical reference points, transverse ligament of hip socket, posterior edge of the hip socket, etc. [26]. The use of transverse ligament helps to define cup position in dysplastic coxarthrosis [27]. Here it is of extreme importance to detect variability of the ligament position according to the gender [28]. Another reference point may be represented with the posterior edge of the hip bone socket, if no expressed bone spurs are observed around it. Some authors suggest using inguinal fold as a reference point to align the cup. During operation the guide for aligning the acetabulum is directed perpendicular to the inguinal fold, which provides for correct inclination setting. The above-mentioned methods are often used for the hip arthroplasty, but they help to define just certain parameters, required to implant alignment, e.g., cup position [29].
ACTIVE CAOS SYSTEMS
The robot-technical CAOS is gaining popularity in the world. First medical research of the hip arthroplasty dates back to the 90ies of previous century. Later, the robots Robodoc and CASPAR® (Orto Maquet, Rastatt, Germany), were created, as well as their modifications, which are beingconstantly improved and modernised. The most widely used robot-engineered systems for hip arthroplasty are the haptic systems, where surgeons physically direct a robotengineered hand through the control desk.
SEMI-ACTIVE CAOS SYSTEMS
In the semi-active system a robot-engineered hand follows the operator hand, holding surgical instruments, it doesn’t move outside the milling trajectory. The systems Acrobot® (Acrobot Company Ltd., London, theUK),system MAKOplasty® (Stryker, Orlando, Florida, the USA), Intellijoint HIP® (Intellijoint Surgical, Inc., Kitchener, Ontario, Canada) and others are represented in the market. Using Acrobot® system, operator directs the system manually by the burr on the robotic lever tip within the milling path, defined according to pre-operational 3D-based planning
THE AUGMENTED REALITY FACILITIES
Another modern technology deals with the augmented technology facilities used during aligning hip arthroplasty components. The Augmented reality (AR) — is projecting any digital information ( image, video, text, graphic image, etc.) over the screen of any facilities. As a result, reality is augmented with artificial elements and new information. The method may be realized using applications to smartphones and tablets, augmented reality glasses, stationary screens, projection facilities and other technologies. There are several other definitions of the augmented reality. In particular, Ronald Azuma in 1997 defined it as a system which: 1) combines virtual and realissues; 2) interacts in real time;3) works with 3D [30].
When using active or semi-active CAOS system, the main interface for the feedback information reflection is within the working field. This means that the surgeon must distribute his attention between the operation field and the screen. The augmented reality technology helps the surgeon to concentrate on the patient, directly providing the viewable feedback information. The surgeon can assess the information feedback, relying on the visual assistance, necessary for precise course of operation. The first steps in this technology use belong to Rodriguez F. (2018) [31] The AR technology can improve accuracy while restoring natural anatomy as well as provide for the better quality control after the final component implantation. Soon the technology would join all the information necessary for a surgeon, the information may be used at all operative stages – from directing the operative access till the implant final position.
DISCUSSION
In patients with well-expressed subcutaneous adipose tissue, registering such points may be complicated and variable, which leads to decrease in accuracy of the passive navigation system [32]. Faulty detection of any bone point by 1 cm leadsto faulty detection by the navigation system of anteversion by 6 ° and inclination - by 2.5 ° [33]. Ultrasound indicators are then used to enhance the accuracy of optical navigation systems [34]. This enhances the implant alignment accuracy, but significantly prolongs surgical intervention ( due to the time spent for attaching the pins and ultrasound study of bone reference points) [35]. As information between the passive navigation system components (marker spheres on the patient, the PC) is transmitted via infrared rays, practice reveals that the surgeons have often been the obstruction, so they often should come far from the operation table in order to provide for correct navigation system functioning, which has rather complicated its use. Contact of the marker spheres with blood or other liquids destroys the navigation system function [36].
The accuracy of implants alignment using active navigation systems makes up about 1 degree or 1 mm [37]. The initial robot-engineered results show improved alignment of acetabular components and decreased occurrence of endoprosthesis head dislocation in early postoperative period. A retrospective study of 2017 which analyzed 300 cases of hip arthroplasty, including 100 robot-engineered procedures, showed 0% dislocation speed in robot-engineered group and 3.0-5.0 % dislocation in ordinary group [38]. As the robot-engineered hip arthroplasty is a relatively new operative intervention method, there are few studies of long-term functional results. The study of 2018, which compared the effectiveness of robot-engineered computer navigation system to ordinary hip arthroplasty with average study period of 14 years has shown insignificant improvement in the robot-engineered group [39].A prospective cohort study of 2019 reports improved accuracy of the robot-engineered system in safe Lewinnek zones compared to the ordinary hip arthroplasty group (96 % against 68 % respectively; p = 0.02). Besides, the study revealed that the use of robot-engineered navigation system was associated with improved restoration of the hip joint center (p <.001) and combined displacement (p <.001)[40]. Evidences prove that the use of robot-engineered computer navigation system may lead to the decrease in speed of early acetabulum component dislocation.
Robotic systems, as well as optical navigation systems, have numerous disadvantages. Their use enhances the implant alignment accuracy, but prolongs the operation duration [41]. Medical robots are huge, which causes certain technical difficulties related to their positioning in the operation room (they obstruct mobility in operating room) [42]. One should mention their high cost, which is another obstacle for their wide use in medical institutions where hip arthroplasty procedure is carried out [43]. The use of the system requires computed tomography examination, which provides for considerable patient exposure to radiation.
In the corpse study Nawabi D.H. et al. [44] have stated that the use of semi-active system MAKOplasty® has shown more accurate cup alignment than manual implantation. Domb B.G. et al. [45] claim that the use of MAKOplasty® system was associated with improved cup alignment in safe Lewinnek zone in 100% cases, compared to 80% in the group where the “free hand” method was used. The advantage of MAKOplasty® system is confirmed by more precise endoprosthesis acetabular component alignment. The disadvantages, mentioned by the authors, are continuous ray exposure of the patient during the CT scanning and high cost of the facilities.
According to literature data, surgeons master the semi-active robot-engineered system faster and easier that the active robot-engineered system. Though, confirmation of compatibility, safety and effectiveness of the method requires for more factual material [46].
The effectiveness of the augmented reality facilities has been assessed in several scientific papers. So, for surgical hip arthroplasty Fotouhi et al. [47] used joined data of real time computer navigation to provide for the cup alignment accuracy, thus reaching low level of error, respectively 1.9 mm, and 0.53 °. Liu H et al. [48] used the data obtained by robot-engineered equipment for drilling the hip guide holes. They compared position and orientation of the drilled holes to pre-operative plan, and the mean inaccuracies were about 2 mm and 2 °. The studies of Hiranaka T. et al. [48] showed that the use of augmented reality facilities during hip arthroplasty provides for increased accuracy as well as prolonged patient radiation exposure and operative intervention duration. Previous results show that the augmented reality may help the surgeon to improve the effectiveness and safety of hip arthroplasty, particularly regarding implants correct alignment [49].
CONCLUSIONS
The views of specialists on optimum position of the hip joint endoprosthesis acetabular component have diverged recently. The most optimum position of the acetabular component is the 45° abduction and 10 -15°anteversion. With posterior access, anteversion should be increased to 20-25 °.
The literature review has shown that computer navigation provide for more accurate cup alignment compared to the traditional method. The surgeons master semi-active robot-engineered system faster and easier than the active robot-engineered one. The authors have confirmed decreased incidence of dislocations and aseptic instability of endoprosthesis components associated with computer navigation used for the hip joint arthroplasty.
Analyzing the use of various computer navigations in total hip arthroplasty, we consider active systems to be the most accurate ones, and semi-active and augmented reality systems for their ease of use.
REFERENCES
1. Wu K.T., Lee P.S., Chou W.Y. et al. Relationship between the social support and self-efficacy for function ability in patients undergoing primary hip replacement. Journal of Orthopaedic Surgery and Research. 2018; 13: 150-5. doi: 10.1186/s13018-018-0857-3.
2. Solarino G., Vicenti G., Piazzolla A. et al. Total hip arthroplasty for dysplastic coxarthrosis using a cementless Wagner Cone stem. Journal of orthopaedics and traumatology: official journal of the Italian Society of Orthopaedics and Traumatology. 2021; 22(1): 16. doi: 10.1186/s10195-021-00578-8.
3. Toonstra J.L., Howell D., English R.A. et al. The Relationship Between Patient Expectations and Functional Outcomes in Patients Undergoing Cartilage Repair of the Knee: A Mixed Methods Study. Journal of Sport Rehabilitation. 2021. doi: 10.1123/jsr.2020-0022.
4. Cevallos N., Soriano K., Flores S.E. et al. Hip Arthroscopy Volume and Reoperations in a Large Cross-Sectional Population: High Rate of Subsequent Revision Hip Arthroscopy in Young Patients and Total Hip Arthroplasty in Older Patients, Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2021. doi: 10.1016/j.arthro.2021.04.017.
5. Pivec R., Johnson A., Mears S.C. Hip arthroplasty. Lancet. 2012; 380 (9855): 1768-77. doi: 10.1016/S0140-6736(12)60607-2.
6. Kurtz Z., Ong K., Lau E. et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030 J. Bone Joint Surg. Am. 2007; 89 (4): 780-5 doi: 10.2106/JBJS.F.00222.
7. Zvereva K.P., Markov D.A., Reshetnikov A.N. Hirurgicheskoe lechenie izolirovannoy asepticheskoy nestabilnosti vertluzhnogo komponenta endoproteza tazobedrennogo sustava. Saratovskiy nauchno-meditsinskiy zhurnal. 2017; 13 (3): 502–6. (in Russian)
8. Mellon S.J., Liddle A.D. Hemant Pandit Hip replacement: Landmark surgery in modern medical history. Maturitas. 2013; 75: 221-6. doi: 10.1016/j.maturitas.2013.04.011.
9. Berend K.R., Sporer S.M., Sierra R.J. Achieving stability and lower-limb length in total hip arthroplasty. J. Bone Joint Surg Am. 2010; 92: 2737-52.
10. Eftekhary N., Shimmin A., Lazennec J.Y. et al. A systematic approach to the hip-spine relationship and its applications to total hip arthroplasty. Bone Joint J. 2019;101-b(7):808–816. doi: 10.1302/0301-620X.101B7.BJJ-2018-1188.R1.
11. Lewinnek G.E., Lewis J.L., Tarr R. et al. Dislocations after total hip-replacement arthroplasties. The Journal of Bone & Joint Surgery. 1978; 60 (2): 217-220. doi: 10.2106/00004623-197860020-00014.
12. Kummer F.J., Shah S., Iyer S., DiCesare P.E. The effect of acetabular cup orientations on limiting hip rotation. J Arthroplasty. 1999;14(4):509-13. doi: 10.1016/s0883-5403(99)90110-9.
13. Ranawac C.S., Maynard M. Modern technique of cemented total hip arthroplasty. Techniques in Orthopaedics. 1991; 6(3): 17-25. doi: 10.1097/00013611-199109000-00004.
14. Sendtner E., Müller M., Winkler R. et al. Femur first beim Hüftgelenksersatz – Das Konzept der kombinierten Anteversion [Femur first in hip arthroplasty--the concept of combined anteversion]. Zeitschrift für Orthopädie und Unfallchirurgie. 2010; 148:185–190. doi: 10.1055/s-0029-1240969.
15. Dorr L.D., Malik A., Dastane M., Wan Z. Combined anteversion technique for total hip arthroplasty. Clin Orthop Relat Res. 2009; 467:119–127. doi: 10.1007/s11999-008-0598-4.
16. Abualkaas I.R. Obosnovanie optimalnogo polozheniya vertluzhnogo komponenta endoproteza tazobedrennogo sustava avtoref. dis. … kand. med. nauk: 14.00.22 SPb. 2003, 20p. (in Russian).
17. Barrack R.L. Dislocation after total hip arthroplasty: implant design and orientation. J Am Acad Orthop Surg. 2003; 11:89–99. doi: 10.5435/00124635-200303000-00003.
18. Weber M., Weber T., Woerner M. et al. The impact of standard combined anteversion definitions on gait and clinical outcome within one year after total hip arthroplasty. Int. Orthop. 2015; 39: 2323–33. doi: 10.1007/s00264-015-2777-8.
19. York P.J., McGee Jr A.W., Dean Ch.S. The relationship of pelvic incidence to post-operative total hip arthroplasty dislocation in patients with lumbar fusion. International Orthopaedics. 2018; 42: 2301–6. doi: 10.1007/s00264-018-3955-2.
20. Di Gioia A.M., Hafez M.A., Jaramaz B. et al. Functional pelvic orientation measured from lateral standing and sitting radiographs. Clin Orthop Relat Res. 2006; 453:272–276. doi: 10.1097/01.blo.0000238862.92356.45.
21. Lembeck B., Mueller O., Reize P., Wuelker N. Pelvic tilt makes acetabular cup navigation inaccurate. Acta Orthop. 2005; 76:517–523. doi: 10.1080/17453670510041501.
22. John A., Perriman D.M., Neeman T.M., Smith P.N. Standing or supine x-rays after total hip replacement – when is the safe zone not safe? Hip International. 2014; 6: 616-23. doi: 10.5301/hipint.5000173.
23. Inaba Y., Kobayashi N., Suzuki H. et al. Preoperative planning for implant placement with consideration of pelvic tilt in total hip arthroplasty: postoperative efficacy evaluation. BMC Musculoskeletal Disorders. 2016; 17: 280-5. doi:10.1186/s12891-016-1120-x.
24. Hasart O., Perka C., Tohtz S. Comparison between pointer-based and ultrasound-based navigation technique in THA using a minimally invasive approach. Orthopedics. 2008;31(10).
25. Ryan J.A., Jamali A.A., Bargar W.L. Accuracy of Computer Navigation for Acetabular Component Placement in THA Clin. Orthop. Relat. Res. 2010; 468:169–77. doi: 10.1007/s11999-009-1003-7.
26. Fukui T., Fukunishi S., Nishio S. et al. Assessment of acetabulum anteversion aligned with the transverse acetabulum ligament: cadaveric study using image-free navigation system. Orthopedic Reviews. 2013; 5: 5. doi: 10.4081/or.2013.e5.
27. Inoue M., Majima T., Abe S. et al. Using the transverse acetabular ligament as a landmark for acetabular anteversion: an intraoperative measurement. Journal of Orthopaedic Surgery. 2013; 21(2): 189. doi: 10.1177/230949901302100215.
28. Epstein S., Woolson N., Giori Epstein N. Acetabular component positioning using the transverse acetabular ligament: Can you find it and does it help? Clin Orthop Relat Res. 2011; 469 (2): 412-16. doi: 10.1007/s11999-010-1523-1.
29. Maillot C., Harman C., Villet L. et al. Modern cup alignment techniques in total hip arthroplasty: A systematic review. Orthop Traumatol Surg Res. 2019 Sep;105(5):907-913. doi: 10.1016/j.otsr.2019.03.015.
30. Azuma R.T. A Survey of Augmented Reality. Presence: Teleoperators and Virtual Environments. August. 1997; 6 (4): 355 - 385. doi: 10.1162/pres.1997.6.4.355.
31. Liu H., Auvinet E., Giles J., Rodriguez F. Augmented reality based navigation for computer assisted hip resurfacing: a proof of concept study. Ann Biomed Eng. 2018; 46(10):1595–605 doi:10.1007/s10439-018-2055-1.
32. Tsukada S., Wakui M. Decreased accuracy of acetabular cup placement for imageless navigation in obese patients. J. Orthop. Sci. 2010; 15: 758–763. doi: 10.1007/s00776-010-1546-1.
33. Hohmann A. Accuracy of acetabular cup positioning using imageless navigation. Journal of Orthopaedic Surgery and Research. 2011; 6: 40-5. doi: 10.1186/1749-799X-6-40.
34. Wassillew G.I. Ultrasound-based computer navigation of the acetabular component: a feasibility study. Arch Orthop Trauma Surg. 2012;.132: 517-25. doi: 10.1007/s00402-011-1412-4.
35. Chaudhry F.A., Ismail S.Z., Davis E.T. New system of computer-assisted navigation leading to reduction in operating time in uncemented total hip replacement in a matched population. European Journal of Orthopaedic Surgery Traumatology. 2018; 28: 645–648. doi: 10.1007/s00590-018-2133-y.
36. Parvizi J., Benson J.R., Muir J.M. A new mini-navigation tool allows accurate component placement during anterior total hip arthroplasty. Medical Devices: Evidence and Research. 2018; 11: 95-104. doi: 10.2147/MDER.S151835.
37. Redmond J.M., Gupta A., Hammarstedt J.E. et al. The learning curve associated with robotic-assisted total hip arthroplasty. The Journal of arthroplasty. 2015; 30: 50–4. doi:10.1016/j.arth.2014.08.003.
38. Illgen R.L., Bukowski B.R., Abiola R. et al. Robotic-Assisted Total Hip Arthroplasty: Outcomes at Minimum Two-Year Follow-Up. Surg Technol Int. 2017; 25(30): 365–372.
39. Bargar W.L., Parise C.A., Hankins A. et al. Fourteen Year Follow-Up of Randomized Clinical Trials of Active Robotic-Assisted Total Hip Arthroplasty. J Arthroplasty. 2018;33(3):810–814. doi:10.1016/j.arth.2017.09.066.
40. Kayani B., Konan S., Thakrar R.R. et al. Assuring the long-term total joint arthroplasty: a triad of variables. Bone Joint J. 2019;101-b(1):11–18. doi: 10.1302/0301-620X.101B1.BJJ-2018-0377.R1.
41. Ahmad Fuad A.N.B., Elangovan H., Deep K., Yao W. A Robotic Flexible Drill and Its Navigation System for Total Hip Arthroplasty. Annals of Biomedical Engineering. 2018; 46 (3): 464–474. doi: 10.1007/s10439-017-1959-5.
42. Putzer D., Klug S., Moctezuma J.L., Nogler M. The Use of Time-of-flight camera for navigating robots in computer-aided surgery: Monitoring the soft tissue envelope of minimally invasive hip approach in a cadaver study. Surgical Innovation. 2014; 21(6): 630 –6. doi: 10.1177/1553350614525669.
43. Sousa P.L., Sculco P.K., Mayman D.J. et al. Robots in the Operating Room During Hip and Knee Arthroplasty. Curr Rev Musculoskelet Med. 2020;13(3):309-317. doi: 10.1007/s12178-020-09625-z.
44. Nawabi D.H., Conditt M.A., Ranawat A.S. et al. Haptically guided robotic technology in total hip arthroplasty: a cadaveric investigation. Proc Inst Mech Eng H. 2013; 227:302–309. doi: 10.1177/0954411912468540.
45. Domb B.G., El Bitar Y.F., Sadik A.Y. et al. Comparison of robotic-assisted and conventional acetabular cup placement in THA: a matched-pair controlled study. Clin Orthop Relat Res. 2014; 472:329–336. doi: 10.1007/s11999-013-3253-7.
46. Sugano N. Computer-assisted orthopaedic surgery and robotic surgery in total hip arthroplasty. Clin Orthop Surg. 2013; 5:1–9. doi:10.4055/cios.2013.5.1.1.
47. Fotouhi J., Alexander C.P., Unberath M. et al. Plan in 2D, execute in 3D: an augmented reality solution for cup placement in total hip arthroplasty. J Med Imaging. 2018; 5(2):1 doi: 10.1117/1.JMI.5.2.021205.
48. Hiranaka T., Fujishiro T., Hida Y. et al. Augmented reality: the use of the PicoLinker smart glasses improves wire insertion under fluoroscopy. World J Orthop. 2017;8(12):891–4. doi:10.5312/wjo.v8.i12.891.
49. Logishetty K., Western L., Morgan R. et al. Can an augmented reality headset improve accuracy of acetabular cup orientation in simulated THA? A randomized trial. Clin Orthop Relat Res. 2019; 477:1190–9.
doi:10.1097/CORR.0000000000000542.
ORCID and contributionship:
Oleksandr A. Haluzynskyi: 0000-0003-2164-4254 A,B,D-F
Volodymyr S. Chornyi : 0000-0002-3679-0783 A,B,D-F
Svitlana V. Burburska: 0000-0002-1487-613X A,B,D
Yevhen V. Kozik: 0000-0002-5839-0334 B,D,E
Conflict of interest:
The Authors declare no conflict of interest.